Recently, the Electrochemical Energy Materials team from the School of Chemistry and Chemical Engineering in GXU has made significant progress in the field of electrocatalytic urea oxidation. Their research findings have been published in Advanced Energy Materials under the title "Regulating the Competitive Adsorption of Urea and OH− via Brønsted Base Intercalated Nickel Sites for Highly Selective Urea Oxidation". The first author of the paper is Jiang Wenjie, a 2022 Ph.D. from the School of Chemistry and Chemical Engineering, with Professor Yin Shibin from the same school serving as the corresponding author. Guangxi University is the sole corresponding affiliation.

Electrocatalytic urea oxidation holds promising application prospects for treating high-concentration urea wastewater and energy conversion due to its advantages of high efficiency, simplicity, environmental friendliness, and low cost. However, under practical urea oxidation conditions, urea molecules and OH− compete for adsorption on the catalyst's active sites, leading to the occurrence of the competing oxygen evolution reaction (OER). This side reaction not only reduces urea oxidation efficiency but also increases energy consumption, posing a key scientific challenge hindering the industrial application of this technology.
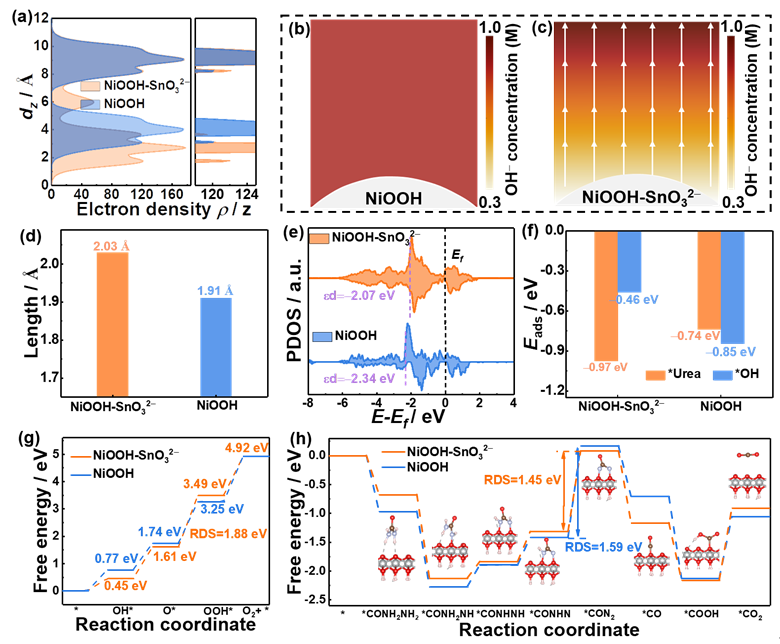
To address this challenge, the research team utilized the intermetallic compound Ni₃Sn₂ as a precursor to construct a catalytic system comprising Brønsted base SnO₃²⁻ intercalated NiOOH (NiOOH-SnO₃²⁻), achieving precise control of the active phase through in-situ surface reconstruction. The study revealed that the SnO₃²⁻ interlayer alters the charge distribution on the NiOOH surface, rendering it negatively charged. This effectively suppresses the strong adsorption of OH− via electrostatic repulsion. Concurrently, the SnO₃²⁻ interlayer stretches the Ni–O bonds and upshifts the d-band center of Ni, thereby enhancing the adsorption and activation capability for urea molecules. This dual effect significantly boosts the urea oxidation reaction rate while markedly suppressing the competing oxygen evolution reaction. This research proposes a novel strategy for achieving highly selective urea oxidation by regulating the competitive adsorption on active sites, offering new insights for the low-energy, high-efficiency treatment of high-concentration urea wastewater.
It is reported that this research received funding and support from the National Natural Science Foundation of China, the Guangxi Natural Science Foundation Key Project, and the High-Performance Computing Platform of Guangxi University. The Electrochemical Energy Materials team, affiliated with the School of Chemistry and Chemical Engineering and the Guangxi Key Laboratory of Electrochemical Energy Materials, has long been dedicated to research and development in electrochemical energy conversion catalysis, chemical energy storage, and graphene functional materials. The team adheres to the "Three-E Principle" – prioritizing "Efficiency, Benefit, and Effectiveness" – and focuses on identifying key scientific issues from practical industrial challenges to solve core technical problems effectively. In the field of electrocatalytic urea oxidation, the team has systematically conducted research on catalytic mechanism elucidation, and catalyst interface structure regulation and design. They have published over 30 academic papers in renowned journals such as Advanced Materials, Advanced Energy Materials, Advanced Functional Materials, ACS Catalysis, Chemical Science, Applied Catalysis B: Environmental, Carbon Energy, Journal of Energy Chemistry, and Nano-Micro Letters, with several papers recognized as ESI Hot Papers and Highly Cited Papers.