Recently, the structural biology team from the School of Life Science and Technology at Guangxi University has achieved new progress in the field of rice immunity and symbiotic signal regulation. The related finding, entitled "Structural basis of OsCERK1-mediated signal activation and transduction in rice immunity and symbiosis," has been published in Plant Communications. Su Zihui, a doctoral student enrolled in 2022 at the School of Life Science and Technology, is the first author of the paper. Professor Ming Zhenhua from the same school is the corresponding author. Guangxi University is listed as the first and corresponding affiliation.
Rice, as a major food crop, faces the complex challenge of resisting pathogen infection while establishing symbiotic relationships with beneficial mycorrhizal fungi during its growth. Chitin elicitor receptor kinase 1 (OsCERK1) is a key protein regulating this balance. However, the molecular mechanism of how it recognizes different signals and activates specific pathways remains unclear, representing a significant scientific question in plant immunity and symbiosis research.
To address this, the research team employed an integrated approach combining structural biology, biochemistry, and functional genetics. They systematically resolved the three-dimensional structures of the intracellular kinase domain of the OsCERK1 protein in nine distinct states. The study revealed that this kinase adopts a unique "intermediate state" conformation. This specific conformation prevents non-specific activation of the signaling pathway in the absence of external stimuli, thereby ensuring the precision of signal transmission.
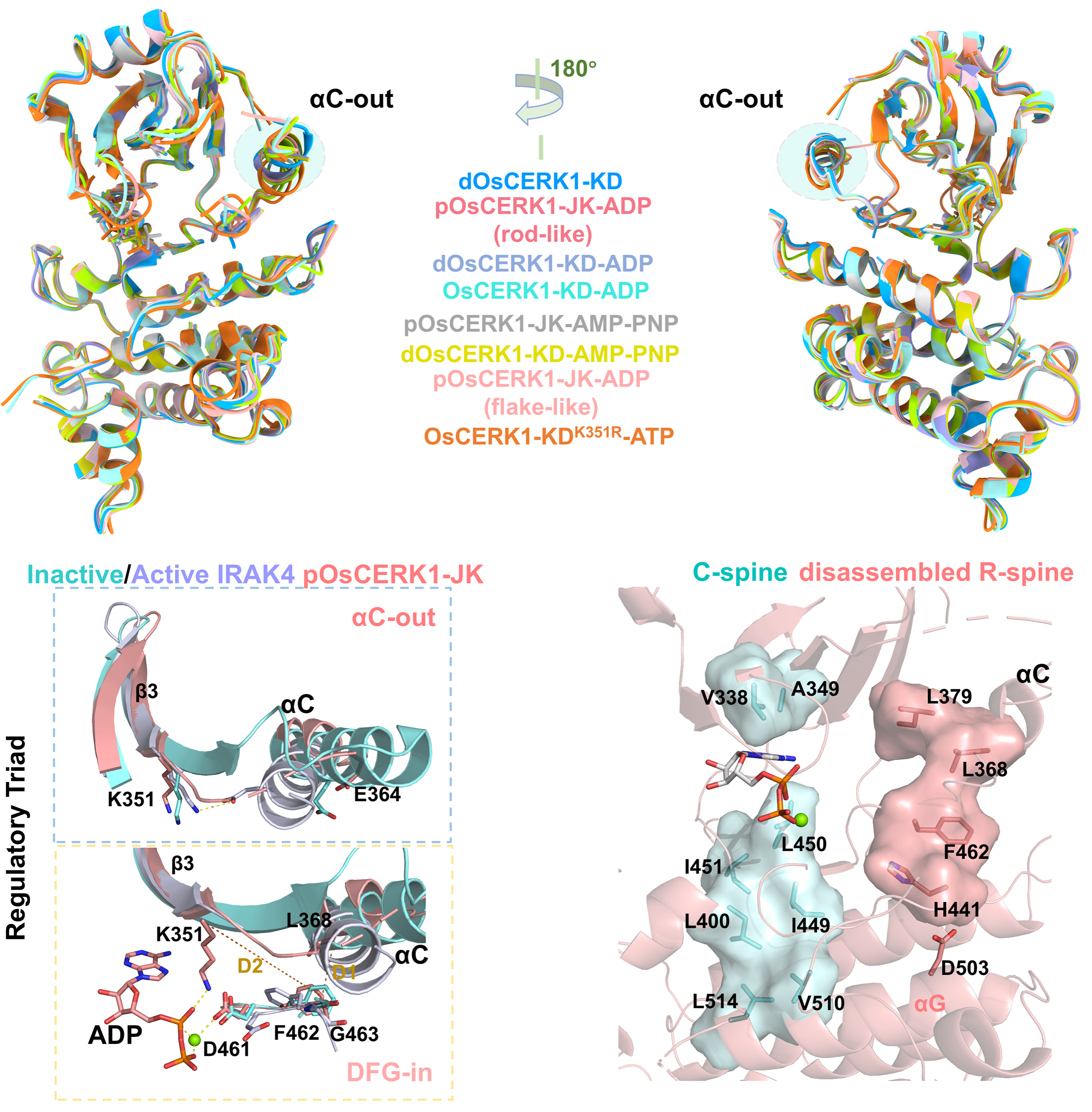
Figure 1 Intermediate-state conformation of the OsCERK1 intracellular kinase domain.
Based on multi-dimensional experimental evidence, the research team has, for the first time, proposed a "dual-regulation model" for OsCERK1 signal transduction. This model suggests that the protein first enhances its catalytic capacity via intermolecular autophosphorylation, and subsequent binding with specific substrate proteins leads to full activation. This finely-tuned regulatory mechanism structurally elucidates how plants utilize the same receptor protein to accurately distinguish between "friend and foe" signals. It not only establishes a new theoretical framework for understanding the coordinated regulation of plant immunity and symbiosis but also provides novel insights for future rational molecular design aimed at improving crop disease resistance and symbiotic efficiency.
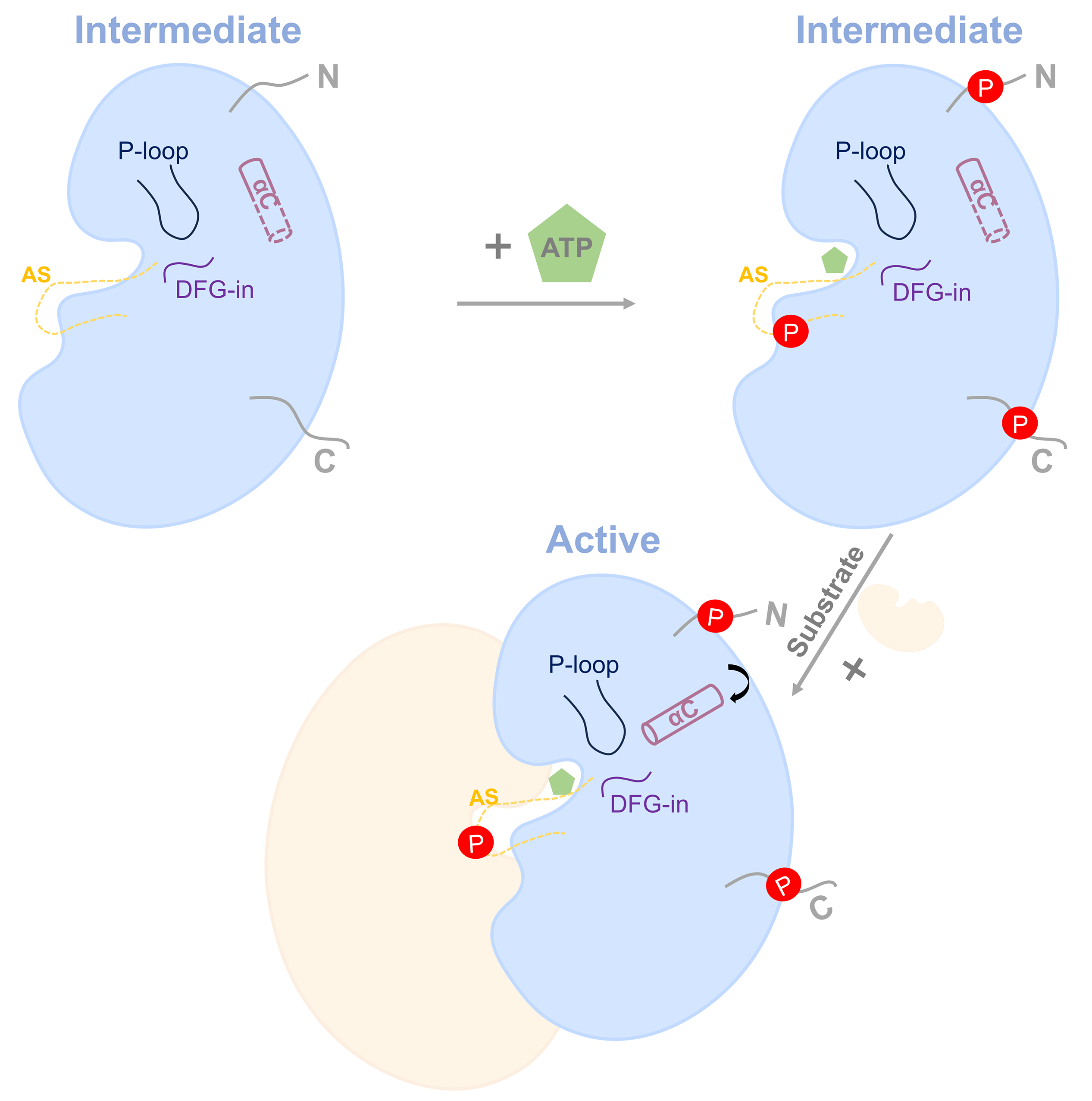
Figure 2 Dual-regulation model of OsCERK1 signal transduction
This achievement marks another advance in plant immune signaling research by the team, following two related studies published consecutively in Plant Communications in 2024. This research was supported by grants from the National Natural Science Foundation of China, the Guangxi Bagui Young Scholars Program, and the Guangxi Distinguished Young Scientists Fund.